12th Chemistry Model Question Paper: வேதியியலில் வெற்றி நிச்சயம்- இதோ பிளஸ் 2 மாதிரி வினாத்தாள்!
TN 12th Chemistry Model Question Paper 2024: 12ஆம் வகுப்பு வேதியியல் பாடத்துக்கான மாதிரி வினாத்தாள் இதோ!

10, 12ஆம் வகுப்பு மாணவர்களுக்கான பொதுத் தேர்வு மாதிரி வினாத்தாள்களை, ABP Nadu ஊடகம் ஆண்டுதோறும் வெளியிட்டு வருகிறது. அந்த வகையில்,12ஆம் வகுப்பு பாடங்களுக்கான மாதிரி வினாத்தாள்களை தினந்தோறும் வெளியிட்டு வருகிறோம்.
12ஆம் வகுப்பு வேதியியல் பாடத்துக்கான மாதிரி வினாத்தாள் இதோ!
MODEL QUESTION PAPER
XII CHEMISTRY
Time:3.00 hours Total Marks:70
PART - I
I.Choose the most suitable answer from the given four alternatives.
1. wolframite ore is separated from tinstone by the process of
a)smelting b)calcination c)Roasting d)Electromagnetic Seperation
2.The element that does not show catenation among the following p-block elements is
a)Carbon b)silicon c) lead d)germarium
3.The basicity of pyrophosphorous acid ( ) is
- a) 4 b)2 c)3 d)5
4.Which one of the following iron has the same number of unpaired electrons as present in
- a) b) c) d)
5.oxidation state of Nickel in [Ni ]
a)2 b)0 c)1 d)3
6.The vacant space in Fcc lattice unit cell is
a)48% b)23% c)32% d)26%
7.After 2 hours, a radioactive substance becomes ( of origin amount .Then the half life (in min)
a)60 minutes b)120 minutes c)30 minutes d)15 minutes
- of a saturated solution of Ca ( is 9.The solubility product (ksp) of Ca
a)0.5 x10-15 b) 0.25x10-10 c)0.125x10-15 d) 0.5x10-10
- which charging lead storage battery
- a) PbSo4 on cathode is reduced to Pb
b)PbSo4on anode is oxidised to Pbo2
- c) PbSo4 on anode is reduced to Pb
d)PbSo4on cathode is oxidised to Pb
10.Milk is colloidal solution of
- a) Solid in gas b)gas is gas c)Liquid in Liquid d)gas in Liquid
- Cold dilutealkaline KMnO4is known is
- a) Schiff ‘s request b) Fehling ‘s request c) Bayer ‘s request d) Nessler ‘s request
12.The formation of cyanohydrin from acetone is an example of
- a) nucleophilicsubstitution b) electrophilic substitution c)electrophilic addition
- d) nucleophilic addition
- which one of the following will not undergo Hofmann bromide reaction?
- a) CH3 CO NHCH3 b) CH3CH2CONH2 c) CH3CONH2 d) C6H5CONH2
- The control dogma of molecular genetics stats that the genetic information flows from:
- a) Amino acids -Protein-DNA b) DNA-Carbohydrates-Protein
c)DNA-RNA-Protein d) DNA-RNA-Carbohydrate
15.Asprin is a/an
- a) acetylsalicylic acid b) benzoyl salicylic acid c) chlorobenzoic acid
- d) anthranilic acid
PART-II
Answer any 6 questions.Question No.24 is compulsory. 6×2=12
- 16. Give the limitations of Ellinghamdiagram?
- It does not explain rate of reaction.
- It does not give any idea about the possibility of other reactions taking place.
- AG is assuming at equilibrium condition, but it is not always true.
- What is burnt alum?
- At475K-500K potash alum loses water of hydration and swells up
- The swollen mass is known as burnt alum.
18.Which is more stable? Fe3+ orFe2+ explain.
Electronic configuration of Fe3+ is [Ar] 3d5
Electronic configuration of Fe2+is[Ar]3d6
Fe3+ is more stable, since it has half-filledorbitals .
19.Why ZnO turns yellow on heating?
On heating ZnO loses oxygen atom and forms a free Zn2+ ion.
This Zn² ion and electrons occupy the interstitial position.
This is due to formation of Metal excess defect.
20.Write a note on catalytic poison?
Substance which decreases the activity of a catalyst
- In the manufacture of ammonia by Haber's process, H₂S acts as a catalytic poison to Fe catalyst
- Give the tests for Carboxylic acids.
Change Blue Litmus paper into Red Colour
Brisk effervescence with Sodium bicarbonate solution
When heated with Alcohol and con. H2SO4. Fruity odour ester is obtained.
22.Write a note on Carbylamine reaction. (Test for primary amines)
Methyl amine + Chloroform Methylisocyanide
CH3NH2 + CHCI3, +3КОН CH3NC + 3KCl + 3H2O
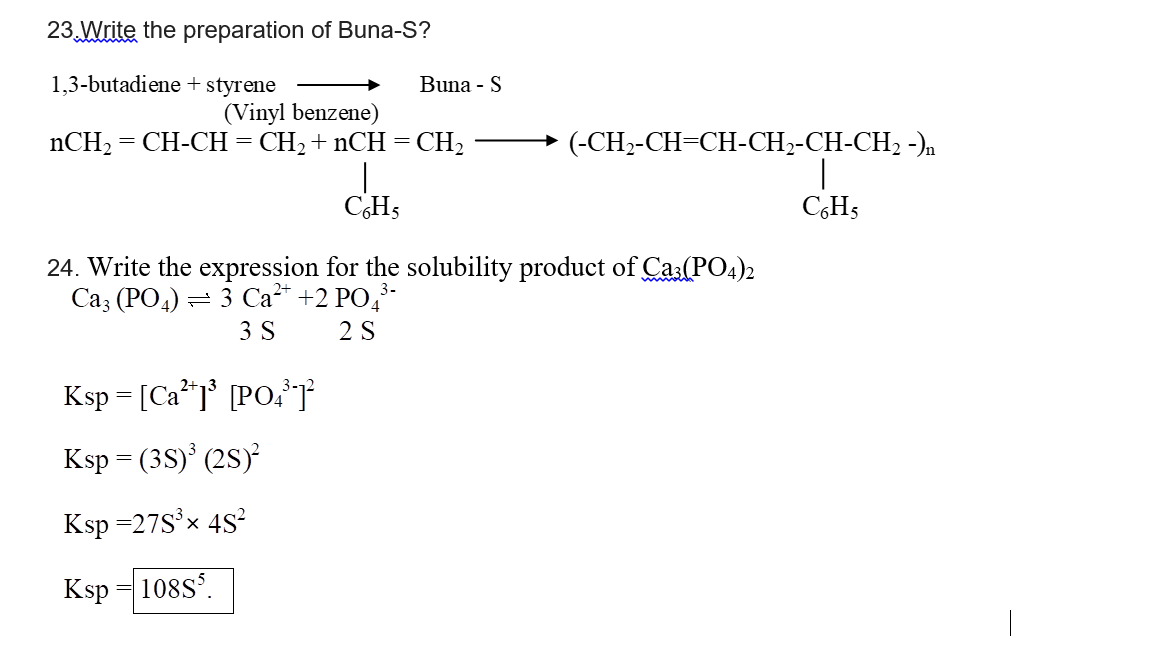
23.Write the preparation of Buna-S?
PART -III
Answer any question.Question No-33 is compulsory 6x3=18
- Differentiate White phosphorus and red phosphorus
| White phosphorus | Red phosphorus |
| Poisonous in nature | It is not poisonous |
| Garlic smell | Odourless |
| It shows Phosphorescence | Does not show Phosphorescence. |
| Its ignition temperature is very low | It does not ignite at low temperatures |
- What is lanthanoid contraction and what are the effects of lanthanoid contraction?
As we move across 4f series, the atomic and ionic radii of Lanthanoids show gradual decrease with increase in atomic number.This decrease in ionic size is called Lanthanoid contraction.
Cause of lanthanoid contraction
The shielding effect of 4f electrons are poor.
Effects of lanthanoid contraction
- Size and radius of ions decreses
- Basicity decreases
- Covalent character increases
The elements of second and third transition series resemble each other more closely.
- A solution of [Ni (H2O)6]2+ is green where as the solution of [Ni (CN)4]2- is colourless explain.
- [Ni (H2O)6]2+ H2O is a weaker ligand. Don't pair d electrons.
Presence of unpaired electron, d-d transaction.Therefore green in colour.
[Ni (CN)4 ]2- CN- is a strong ligand, d electrons are paried.
Absence of unpaired electron. No d-d transaction. Therefore colourless.

29.Define std hydrogen Electrode (SHE)
The emf of a cell is the sum of the electrode potentials at the cathode and anode,
Ecell = (Eox) anode + (Ered)cathode
- Standard Hydrogen Electrode (SHE) is used as the reference electrode.
Emf is zero volt.
- It consists of a platinum electrode in contact with IM HCI solution and 1 atm hydrogen gas.
- The hydrogen gas is bubbled through the solution at 25°C
- SHE can act as a cathode as well as an anode.
The Half cell reactions are given below
- Anode - oxidation
H2 2H+ + 2e- E° = 0 v
- Cathode reduction
2H+ + 2 H2 E° = 0 v
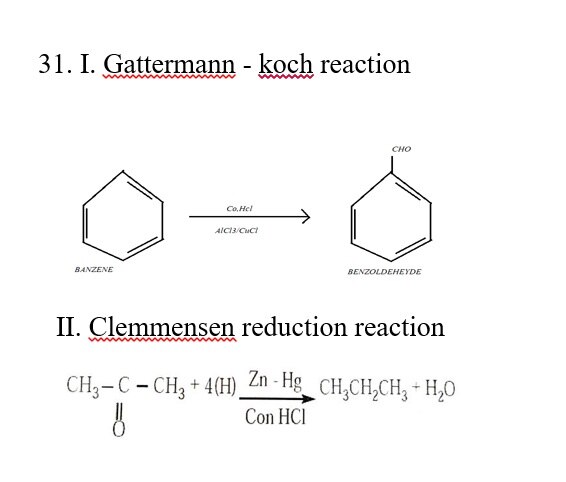
30.Expalin intermediate compound formation theory of catalysis with an example?
It is based on a homogeneous catalysed reaction
A+B AB
Step -I : A+C (intermediate compound)
Step -II: AC+B
Example
2SO2(g) +O2(g) 2SO3
Step-I: 2NO +O2 2NO2
Step-II: NO2+SO2 SO3+NO
- Write a short note on peptide bond
The carboxyl group of the first amino acid react with the amino group of the second amino acid to form peptide bond.
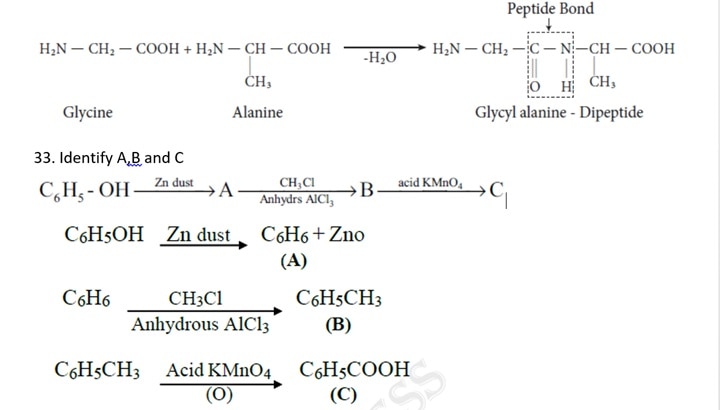
- Identify A,B and C
PART-IV
Answer all the questions
34. a)
- i) Explain the Magnetic separation process (3)
- It is based on the difference in the Magnetic properties of the ore and the impurities
- It is used to concentrate ferromagnetic ores.
- Tin stone can be separated from the wolframite impurities.
- The powered ore is added on an electro magnet containing a moving belt on a magnetic rollers.
- The magnetic ore falls near the magnet.
- The non magnet parts fall away from the magnet.
ii) Why HF is not stored in glass bottles? (2)
Monist hydrofluoric acid HF(not dry ) rapidly react with sodium silicate in glass.
SiO2+4HF SiF4+2H2O
Na2SiO3+6HF Na2SiF6+3H2O
OR
b)Write a note on zeolites? (3)
- Zeolites are three dimensional crystalline solids containing aluminium,silicon and oxygen.
- Si and Al atoms are tetrahedrally coordinated with oxygen atoms .
- They are Hydrated solidum alumino silicate (NaO.Al2O3.xSiO2.yH2O).
- They have porous structure in which the monovalent sodium ions and water molecules are loosely held.
- Water molecules move freely in and out of these pores.
- They have crystalline structure looks like a honeycomb consisting of a network of interconnected tunnels and cages.
- The crystal to act as a molecular sieve.
ii)Write the oxidation state ,co-ordination number Ligand central metal atom in K4[Fe(CN)6] complex (2)
Complex K4[Fe(CN)6]
Oxidation state +2
Co ordination number 6
Ligand CN-
Central metal atom Fe2+
35.a) Calculate the percentage efficiency of packing in case of body centered cubic crystal. (5)

- B. Write the pastulates of werner’s theory. (5)
The central metal atom exhibit two type of velence.
- Primary valency secondary valency.
|
PRIMARY VALENCY |
SECONDARY VALENCY |
|
Oxidation number of the central metal ion |
Coordination number of the central metal ion |
|
It is non directional |
It is directional |
|
Ionisable valency |
Non Ionisable valency |
|
Always satisfied by negative ions |
Satisfied by negative ions OR neutral molecules OR positive ions |
There are two spheres of attraction around a metal atom / ion in a complex.
- The inner sphere (coordination sphere)
The group present in this sphere are firmly attached to the central metal ion .
2.The out sphere (ionisation sphere)
The group present in this sphere are loosly attached to the central metal ion .
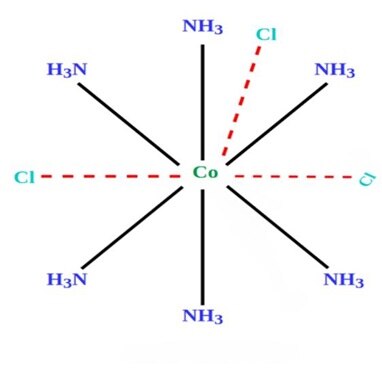
Structure of compound [Co(NH3]Cl3
Limitation of werner’s theory.
It does not explain the colour and magnetic properties of coordination compounds.
- a) I) Derive henderson - hasselbatch quation (3)
HA + H2O = [H3O+] = (acid)eq
(base)eq
Due to common ion effect
(acid)eq = (acid) : (base)eq = (salt)
[H3O+] = Ka (acid) / ( salt )
Revers the sign on both sides
-log [H3O+] = -log Ka -log (acid) / ( salt )
We know that
PH = - -log [H3O+] and pKa = -log Ka
PH = pKa - log (acid) / ( salt )
PH = pKa + log (acid) / ( salt )
Similarly for absic buffer
POH = pKb + log (acid) / ( salt )
- ii) write a note on sacrificial protection. (2)
sacrificial protection (cathodic prodection)
- sacrificial anode - zinc
- Cathode - iron
- So iron is prodected,but Zn is corroded
(or)
- b) Explain dispersion methods of preparation of colloids. (5)
- mechanical dispersion
- The colloidal mill corists of two metal places roating in opposite direction at very high speed.
- The solid is ground to colloidal dimension.
- Colloidal solutions of onk and grapite as pepared.
- Peptisation
The disperson of a precipitated matirial into colloidal solution by the action of an electrolyte in solution is termed as peptisation.
Hcl
Agcl Agcl
Precipitate collide
- a) i. How will you distinguish between primary,secondary and tertiary amines? (3)
|
Reagent |
Primary amines |
Secondary amines |
Tertiary amines |
| Nitrous acid | Forms alcohol | Forms N-nitroso amine | Forms salt |
| Carbylamine reaction CHCl3 / KOH | Forms Carbylamine | No rection | No rection |
| Mustard oil rection CS2 / Hgcl2 | Forms alkyl isothiocynate | No rection | No rection |
| Acetyl chloride | Forms N-alkyl acetinide | Forms N,N-do alkyl acetamide | No rection |
| Alkyl halides | 3 molecules of alkyl halide,quarternary ammonium salt is formed | 2 molecules of alkyl halide,quarternary ammonium salt is formed | 1 molecules of alkyl halide,quarternary ammonium salt is formed |
- ii) Write the uses of diethyl ether. (2)
- Anesthetic agent in surgery
- Used as a refrigerant
- Solvent for organic reactions.
(or)
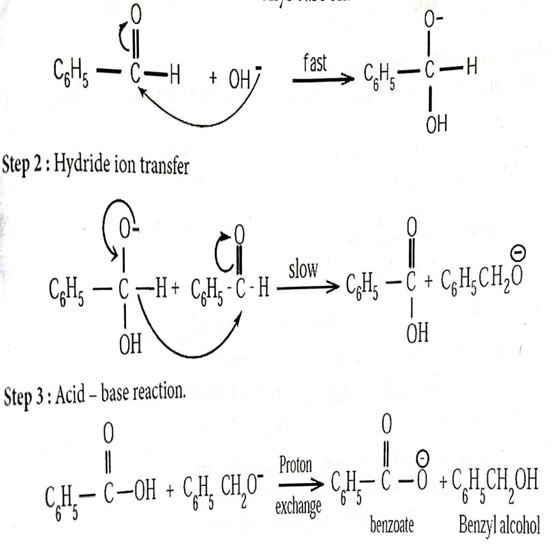
- b) Mechanism of cannizaro reaction (5)
Cannizaro reaction is a characteristic reaction of aldehyde having na α
- hydrogen
Dil.NaOH
C6H5CHO+C6H5CHO -------> C6H5CH2OH+ C6H5COONa
Step : 1 Attack of OH- On the carbonyl carbon.
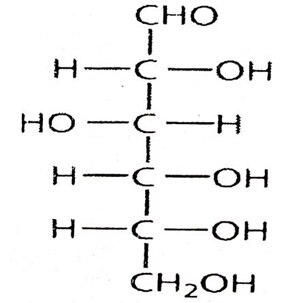
38. a). Elucidate the structure of glucose (5)
- Molecular formula C6H12O6
- Glucose + P/H1 At 373k n-hexane.6 carbon atoms are bonding linearly
- Glucose + HCN cyanohydrins presence of carbonyl group.
- Glucose + acetic anhydride + pyridine penta acetate. It contains 5 OH groups
- Glucose + tollens reagent reduction to metalic silver.presence of aldehyde group
(or)
b). i) Write a note on vulcanization of rubber. (3)
- Natural rubber is mixed 3-5% sulphur and heated at 100- 1500 c causes cross linking of the cis - 1,4-polyisopreene chains through disulphide (-s-s-) bonds.
- The properties of natural rubber can be modified by the process called vulcanization.
- 1 to 3% sulphur rubber is soft and stretchy
- 3 to 10% sulphur rubber is harder flexible.
- ii) How the tranquilizers work in body? (2)
- They are neurolgically active drugs.
- They acts on the central nervous system by blocking the neuro transtmitter dopomine in the brain.
- The treatment of stress,sleep disorders and severe mental disease.
- Major tranquilizers - haloperidol,clozapine
- Minor tranquilizers - valium,alprazolam
இதையும் வாசிக்கலாம்: 12th Physics Model Question Paper: இனி இயற்பியலும் இனிமைதான்- பிளஸ் 2 மாதிரி வினாத்தாள் இதோ!



































